vsepr model chart|printable vsepr chart : iloilo Postulates. The central atom in polyatomic molecules is surrounded by several other atoms. A polyatomic molecule consists of three or more atoms. The total number of valence shell electron pairs determines the .
Students of the Ateneo de Manila University (AdMU) on Tuesday staged a protest on campus amid allegations of sexual misconduct by teaching staff that recently went viral on social media. The protest in front of the Horacio de la Costa Hall, where the accused professors' offices were located, was led by Philosophy instructor Luther .
PH0 · vsepr structures chart
PH1 · vsepr shape chart
PH2 · vsepr model generator
PH3 · vsepr model examples
PH4 · vsepr geometry table
PH5 · vsepr chart with bond angles
PH6 · vsepr angle chart
PH7 · printable vsepr chart
PH8 · Iba pa
3. Thunderbolt Casino: 50 free spins on Plentiful Treasure. New SA players can begin their gambling journeys with a bang by claiming 50 free spins on Thunderbolt Casino's popular slot, Plentiful Treasure, with no deposit required. This bonus comes with a 60x wagering requirement, so you must wager R,3000 (60 x 50) before your winnings can be withdrawn.
vsepr model chart*******The valence-shell electron-pair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases. It is based on the assumption .
The VSEPR model is useful for predicting and visualizing molecular structures. The structures are: linear, trigonal planar, angled, tetrahedral, trigonal pyramidal, trigonal .
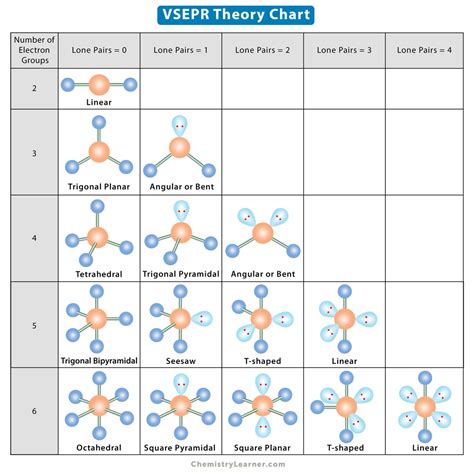
VSEPR theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. A central atom is defined in this theory as an atom which is bonded to two or more other atoms, while a terminal atom is bonded to only one other atom. For example in the molecule methyl isocyanate (H3C-N=C=O), the two carbons an.Learn how to use the valence shell electron pair repulsion (VSEPR) model to predict the 3-D shape of molecules and ions. Find the electron pair geometry, molecular geometry, and bond angle for each compound, .printable vsepr chart What is VSEPR theory? Learn the postulates of VSEPR theory and the application of VSEPR theory in predicting the shapes of molecules. Also, see the VSEPR chart. Updated: 11/21/2023
Postulates. The central atom in polyatomic molecules is surrounded by several other atoms. A polyatomic molecule consists of three or more atoms. The total number of valence shell electron pairs determines the .The valence-shell electron-pair repulsion (VSEPR) model allows us to predict which of the possible structures is actually observed in most cases. It is based on the assumption that pairs of electrons occupy space, and .
Introduction. The shapes of the molecules is determined mainly by the electrons surrounding the central atom. Therefore, VSEPR theory gives simple directions on how .VSEPR. Model. VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL. Lewis structures show the two-dimensional distribution of atoms and electrons.VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .
Molecular Models (VSEPR Theory) - University of Illinois Urbana-Champaign . NextVSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .
vsepr model chart printable vsepr chartWe recommend using the latest version of Chrome, Firefox, Safari, or Edge. Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) MODEL. Lewis structures show the two-dimensional distribution of atoms and electrons. The molecular geometry, or three-dimensional shape of a molecule or polyatomic ion, can be determined using valence-shell electron-pair repulsion (abbreviated VSEPR and pronounced “VES-per”) theory, in .We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, .
VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .
VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .The VSEPR model combines the original ideas of Sidwick and Powell and further development of Nyholm and Gillespie. How VSEPR works. In a molecule EX n, the valence shell electron pair around the central atom E and the E-X single bonds are very important due to the repulsion in which determine the shape of the molecule. The repulsions .vsepr model chartAus dem VSEPR-Modell ergeben sich abgeleitete Regeln, wobei folgende Abkürzungen verwendet werden: A: Zentralatom B: bindende Elektronenpaare E: freie Elektronenpaare. In Molekülen des Typs AB n .

The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The VSEPR model is not a theory; it does not attempt to explain observations. Instead, it is a counting procedure that accurately .
Use our handy VSEPR chart to find the 3-D geometric VSEPR shapes of molecules and ions. Learn about VSEPR theory and shapes like trigonal planar or square pyramidal. . Under the VSEPR model, a trigonal bipyramidal molecule such as phosphorus pentachloride or PCl 5, with a central phosphorus atom and five valence shell electron .
According to VSEPR theory, a molecule is designated by the letters AX m E n. “A” represents the central atom, “X” represents the bonded atoms, “E” represents the lone pairs on the central atom, “m” is the number of electron groups or domains, and “n” is the number of lone pairs on the central atom. Example: The water (H 2 O .VSEPR is an acronym that stands for valence shell electron pair repulsion. The model was proposed by Nevil Sidgwick and Herbert Powell in 1940. Ronald Gillespie and Ronald Nyholm then developed the model into their theory published in 1957; they are considered the developers of the VSEPR theory. The approach was commonly referred to as .
Introduction. This section explores how we predict the molecular and electron-pair shapes of molecules using the VSEPR (Valence Shell Electron Pair Repulsion) theory. We will first go over what VSEPR theory is and how it defines an electron-pair geometry and a molecular geometry. Then we will go over the steps for determining the electron-pair .Figure 9.2.1 9.2. 1: Common Structures for Molecules and Polyatomic Ions That Consist of a Central Atom Bonded to Two or Three Other Atoms. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.
Check 'sobra' translations into English. Look through examples of sobra translation in sentences, listen to pronunciation and learn grammar. . sinasabing sangkatlo ng lahat ng taga-Hilagang Amerika ay sobra sa timbang o sobrang taba. . kulang sa suweldo, pagod sa paglipat-lipat sa mga pasyente, kakaunti ang gamot, walang ospital, kakaunti .
vsepr model chart|printable vsepr chart